The Compound Ch3 - Ch2 - Sh Is in the Organic Family Known as

Thiol with a blue highlighted sulfhydryl group.
A thiol ()[i] or thiol derivative is any organosulfur compound of the grade R−SH, where R represents an alkyl or other organic substituent. The –SH functional group itself is referred to as either a thiol group or a sulfhydryl group, or a sulfanyl grouping. Thiols are the sulfur counterpart of alcohols (that is, sulfur takes the place of oxygen in the hydroxyl group of an alcohol), and the word is a blend of "thio-" with "alcohol", where the first word deriving from Greek θεῖον (theion) significant "sulfur".[2]
Many thiols have potent odors resembling that of garlic or rotten eggs. Thiols are used equally odorants to assist in the detection of natural gas (which in pure form is odorless), and the "smell of natural gas" is due to the smell of the thiol used as the odorant. Thiols are sometimes referred to as mercaptans.[3] [4] [5] The term "mercaptan" [6] was introduced in 1832 by William Christopher Zeise and is derived from the Latin mercurio captāns (capturing mercury)[7] because the thiolate group (RS−) bonds very strongly with mercury compounds.[viii]
Construction and bonding [edit]
Thiols of the structure R-SH are referred to as Alkanethiols or Alkyl thiols, in which an alkyl group (R) is attached to a sulfhydryl group (SH).[nine] Thiols and alcohols take similar connectivity. Because sulfur atoms are larger than oxygen atoms, the C−S bond lengths – typically around 180 picometres in length – are about 40 picometers longer than a typical C−O bond. The C−S−H angles approach xc° whereas the bending for the C−O−H grouping is more obtuse. In the solid or liquids, the hydrogen-bonding betwixt individual thiol groups is weak, the main cohesive force being Van der Waals interactions between the highly polarizable divalent sulfur centers.
The S−H bond is much weaker than the O−H bail as reflected in their respective bail dissociation energy (BDE). For CH3S−H, the BDE is 366 kJ/mol (87 kcal/mol), while for CH3O−H, the BDE is 440 kJ/mol (110 kcal/mol).[10]
Due to the pocket-size departure in the electronegativity of sulfur and hydrogen, an Due south−H bond is moderately polar. In dissimilarity, O−H bonds in hydroxyl groups are more polar. Thiols take a lower dipole moment relative to their corresponding alcohols.
Nomenclature [edit]
There are several ways to proper name the alkylthiols:
- The suffix -thiol is added to the name of the alkane. This method is well-nigh identical to naming an alcohol and is used by the IUPAC, due east.g. CH3SH would be methanethiol.
- The word mercaptan replaces alcohol in the name of the equivalent alcohol compound. Example: CH3SH would be methyl mercaptan, just equally CH3OH is called methyl alcohol.
- The term sulfhydryl- or mercapto- is used equally a prefix, e.g. mercaptopurine.
Physical properties [edit]
Aroma [edit]
Many thiols have strong odors resembling that of garlic. The odors of thiols, especially those of depression molecular weight, are often strong and repulsive. The spray of skunks consists mainly of low-molecular-weight thiols and derivatives.[11] [12] [13] [fourteen] [15] These compounds are detectable by the human nose at concentrations of only 10 parts per billion.[16] Human being sweat contains (R)/(S)-3-methyl-3-mercapto-ane-ol (MSH), detectable at 2 parts per billion and having a fruity, onion-like odor. (Methylthio)methanethiol (MeSCHtwoSH; MTMT) is a strong-smelling volatile thiol, also detectable at parts per billion levels, found in male mouse urine. Lawrence C. Katz and co-workers showed that MTMT functioned as a semiochemical, activating certain mouse olfactory sensory neurons, attracting female mice.[17] Copper has been shown to be required past a specific mouse olfactory receptor, MOR244-three, which is highly responsive to MTMT as well equally to various other thiols and related compounds.[18] A human being olfactory receptor, OR2T11, has been identified which, in the presence of copper, is highly responsive to the gas odorants (see beneath) ethanethiol and t-butyl mercaptan also as other depression molecular weight thiols, including allyl mercaptan found in human garlic jiff, and the stiff-smelling cyclic sulfide thietane.[xix]
Thiols are besides responsible for a class of wine faults acquired by an unintended reaction between sulfur and yeast and the "skunky" odor of beer that has been exposed to ultraviolet lite.
Not all thiols have unpleasant odors. For example, furan-2-ylmethanethiol contributes to the aroma of roasted java, whereas grapefruit mercaptan, a monoterpenoid thiol, is responsible for the characteristic scent of grapefruit. The effect of the latter compound is present only at low concentrations. The pure mercaptan has an unpleasant odour.
Natural gas distributors were required to add thiols, originally ethanethiol, to natural gas (which is naturally odorless) after the deadly New London School explosion in New London, Texas, in 1937. Many gas distributors were odorizing gas prior to this event. Most gas odorants utilized currently contain mixtures of mercaptans and sulfides, with t-butyl mercaptan every bit the chief odor constituent in natural gas and ethanethiol in liquefied petroleum gas (LPG, propane).[20] In situations where thiols are used in commercial industry, such as liquid petroleum gas tankers and majority handling systems, an oxidizing catalyst is used to destroy the odor. A copper-based oxidation catalyst neutralizes the volatile thiols and transforms them into inert products.
Boiling points and solubility [edit]
Thiols testify little association past hydrogen bonding, both with h2o molecules and among themselves. Hence, they have lower boiling points and are less soluble in h2o and other polar solvents than alcohols of similar molecular weight. For this reason also, thiols and their corresponding sulfide functional grouping isomers have similar solubility characteristics and boiling points, whereas the aforementioned is non true of alcohols and their respective isomeric ethers.
Bonding [edit]
The S−H bond in thiols is weak compared to the O−H bond in alcohols. For CH310−H, the bond enthalpies are 365.07±2.1 kcal/mol for X = South and 440.2±3.0 kcal/mol for 10 = O.[21] Hydrogen-atom abstraction from a thiol gives a thiyl radical with the formula RS•, where R = alkyl or aryl.
Characterization [edit]
Volatile thiols are easily and almost unerringly detected by their distinctive odor. Sulfur-specific analyzers for gas chromatographs are useful. Spectroscopic indicators are the DiiO-exchangeable SouthwardH signal in the 1H NMR spectrum (33Southward is NMR-active but signals for divalent sulfur are very broad and of little utility[22]). The ν SH band appears nigh 2400 cm−1 in the IR spectrum.[3] In the nitroprusside reaction, costless thiol groups react with sodium nitroprusside and ammonium hydroxide to give a red colour.
Preparation [edit]
In industry, methanethiol is prepared past the reaction of hydrogen sulfide with methanol. This method is employed for the industrial synthesis of methanethiol:
- CHiiiOH + H2S → CH3SH + HiiO
Such reactions are conducted in the presence of acidic catalysts. The other main route to thiols involves the improver of hydrogen sulfide to alkenes. Such reactions are usually conducted in the presence of an acrid goad or UV light. Halide displacement, using the suitable organic halide and sodium hydrogen sulfide has also been utilized.[23]
Another method entails the alkylation of sodium hydrosulfide.
- RX + NaSH → RSH + NaX(10 = Cl, Br, I)
This method is used for the production of thioglycolic acid from chloroacetic acid.
Laboratory methods [edit]
In general, on the typical laboratory scale, the directly reaction of a haloalkane with sodium hydrosulfide is inefficient owing to the competing formation of sulfides. Instead, alkyl halides are converted to thiols via an S-alkylation of thiourea. This multistep, i-pot procedure proceeds via the intermediacy of the isothiouronium table salt, which is hydrolyzed in a separate step:[24] [25]
- CH3CH2Br + SC(NHtwo)two → [CH3CH2SC(NH2)2]Br
- [CH3CHiiSC(NH2)2]Br + NaOH → CHiiiCH2SH + OC(NH2)2 + NaBr
The thiourea road works well with chief halides, especially activated ones. Secondary and tertiary thiols are less easily prepared. Secondary thiols can be prepared from the ketone via the corresponding dithioketals.[26] A related 2-step process involves alkylation of thiosulfate to give the thiosulfonate ("Bunte salt"), followed by hydrolysis. The method is illustrated past one synthesis of thioglycolic acid:
- ClCHtwoCOiiH + NaiiSouthward2Oiii → Na[O3S2CHiiCO2H] + NaCl
- Na[O3Due south2CHtwoCOiiH] + HtwoO → HSCH2COiiH + NaHSO4
Organolithium compounds and Grignard reagents react with sulfur to give the thiolates, which are readily hydrolyzed:[27]
- RLi + South → RSLi
- RSLi + HCl → RSH + LiCl
Phenols tin exist converted to the thiophenols via rearrangement of their O-aryl dialkylthiocarbamates.[28]
Thiols are prepared by reductive dealkylation of sulfides, especially benzyl derivatives and thioacetals.[29]
Thiophenols are produced by Due south-arylation or the replacement of diazonium leaving group with sulfhydryl anion (SH−):[30] [31]
- ArN +
2 + SH− → ArSH + N2
Reactions [edit]
Akin to the chemical science of alcohols, thiols form sulfides, thioacetals, and thioesters, which are analogous to ethers, acetals, and esters respectively. Thiols and alcohols are also very different in their reactivity, thiols being more hands oxidized than alcohols. Thiolates are more strong nucleophiles than the respective alkoxides.
Southward-Alkylation [edit]
Thiols, or more specific their conjugate bases, are readily alkylated to give sulfides:
- RSH + R′Br + B → RSR′ + [HB]Br (B = base)
Acidity [edit]
Thiols are easily deprotonated.[32] Relative to the alcohols, thiols are more acidic. The conjugate base of a thiol is called a thiolate. Butanethiol has a p1000 a of 10.5 vs 15 for butanol. Thiophenol has a pK a of 6, versus 10 for phenol. A highly acidic thiol is pentafluorothiophenol (C6FvSH) with a pK a of ii.68. Thus, thiolates can be obtained from thiols past treatment with element of group i hydroxides.

Synthesis of thiophenolate from thiophenol
Redox [edit]
Thiols, peculiarly in the presence of base, are readily oxidized past reagents such as bromine and iodine to requite an organic disulfide (R−S−S−R).
- 2 R−SH + Br2 → R−S−S−R + 2 HBr
Oxidation by more powerful reagents such as sodium hypochlorite or hydrogen peroxide can also yield sulfonic acids (RSO3H).
- R−SH + 3 H2O2 → RSO3H + 3 H2O
Oxidation can also be effected by oxygen in the presence of catalysts:[33]
- 2 R–SH + ane⁄ii O2 → RS−SR + HiiO
Thiols participate in thiol-disulfide exchange:
- RS−SR + 2 R′SH → 2 RSH + R′Due south−SR′
This reaction is important in nature.
Metal ion complexation [edit]
With metal ions, thiolates bear as ligands to grade transition metal thiolate complexes. The term mercaptan is derived from the Latin mercurium captans (capturing mercury)[7] considering the thiolate group bonds so strongly with mercury compounds. According to hard/soft acid/base (HSAB) theory, sulfur is a relatively soft (polarizable) atom. This explains the tendency of thiols to bind to soft elements and ions such as mercury, pb, or cadmium. The stability of metal thiolates parallels that of the corresponding sulfide minerals.
Thioxanthates [edit]
Thiolates react with carbon disulfide to give thioxanthate (RSCS −
2 ).
Thiyl radicals [edit]
Costless radicals derived from mercaptans, called thiyl radicals, are commonly invoked to explain reactions in organic chemical science and biochemistry. They have the formula RS• where R is an organic substituent such as alkyl or aryl.[five] They ascend from or can be generated past a number of routes, only the principal method is H-atom abstraction from thiols. Another method involves homolysis of organic disulfides.[34] In biological science thiyl radicals are responsible for the formation of the deoxyribonucleic acids, edifice blocks for Deoxyribonucleic acid. This conversion is catalysed past ribonucleotide reductase (see figure).[35] Thiyl intermediates as well are produced by the oxidation of glutathione, an antioxidant in biology. Thiyl radicals (sulfur-centred) can transform to carbon-centred radicals via hydrogen atom commutation equilibria. The formation of carbon-centred radicals could pb to poly peptide harm via the formation of C−C bonds or backbone fragmentation.[36]
Because of the weakness of the Due south−H bail, thiols can functioning equally scavengers of free radicals.[37]
Biological importance [edit]
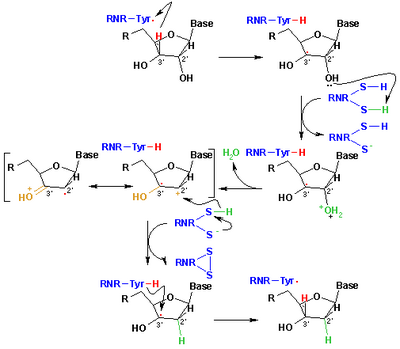
The catalytic cycle for ribonucleotide reductase, demonstrating the part of thiyl radicals in producing the genetic machinery of life.
Cysteine and cystine [edit]
As the functional grouping of the amino acid cysteine, the thiol group plays a very important function in biology. When the thiol groups of two cysteine residues (every bit in monomers or constituent units) are brought nearly each other in the course of protein folding, an oxidation reaction can generate a cystine unit of measurement with a disulfide bond (−S−Due south−). Disulfide bonds can contribute to a poly peptide's 3rd structure if the cysteines are part of the aforementioned peptide chain, or contribute to the 4th structure of multi-unit proteins past forming fairly potent covalent bonds betwixt different peptide chains. A physical manifestation of cysteine-cystine equilibrium is provided past hair straightening technologies.[38]
Sulfhydryl groups in the active site of an enzyme tin can grade noncovalent bonds with the enzyme's substrate too, contributing to covalent catalytic activeness in catalytic triads. Active site cysteine residues are the functional unit in cysteine protease catalytic triads. Cysteine residues may also react with heavy metal ions (Zn2+, Cd2+, Pbii+, Hg2+, Ag+) because of the high analogousness between the soft sulfide and the soft metal (see hard and soft acids and bases). This can deform and inactivate the poly peptide, and is one mechanism of heavy metal poisoning.
Drugs containing thiol group 6-Mercaptopurine (anticancer) Captopril (antihypertensive) D-penicillamine (antiarthritic) Sodium aurothiolate (antiarthritic)[39]
Cofactors [edit]
Many cofactors (non-protein-based helper molecules) feature thiols. The biosynthesis and degradation of fatty acids and related long-chain hydrocarbons is conducted on a scaffold that anchors the growing chain through a thioester derived from the thiol Coenzyme A. The biosynthesis of methane, the principal hydrocarbon on World, arises from the reaction mediated past coenzyme M, 2-mercaptoethyl sulfonic acid. Thiolates, the conjugate bases derived from thiols, form strong complexes with many metal ions, peculiarly those classified equally soft. The stability of metal thiolates parallels that of the corresponding sulfide minerals.
In skunks [edit]
The defensive spray of skunks consists mainly of depression-molecular-weight thiols and derivatives with a foul smell, which protects the skunk from predators. Owls are able to prey on skunks, equally they lack a sense of smell.[xl]
Examples of thiols [edit]
- Methanethiol – CHthreeSH [methyl mercaptan]
- Ethanethiol – CtwoH5SH [ethyl mercaptan]
- ane-Propanethiol – C3H7SH [n-propyl mercaptan]
- two-Propanethiol – CHiiiCH(SH)CHthree [2C3 mercaptan]
- Allyl mercaptan – CHtwo=CHCH2SH [2-propenethiol]
- Butanethiol – C4H9SH [n-butyl mercaptan]
- tert-Butyl mercaptan – (CH3)3CSH [t-butyl mercaptan]
- Pentanethiols – CvHelevenSH [pentyl mercaptan]
- Thiophenol – CsixH5SH
- Dimercaptosuccinic acid
- Thioacetic acid
- Coenzyme A
- Glutathione
- Metallothionein
- Cysteine
- 2-Mercaptoethanol
- Dithiothreitol/dithioerythritol (an epimeric pair)
- ii-Mercaptoindole
- Grapefruit mercaptan
- Furan-2-ylmethanethiol
- 3-Mercaptopropane-1,2-diol
- iii-Mercapto-1-propanesulfonic acid
- i-Hexadecanethiol
- Pentachlorobenzenethiol
See also [edit]
- Persulfide
- Doctor sweetening process
- Odorizer
- Saville reaction
- Thiol-disulfide exchange
References [edit]
- ^ Dictionary Reference: thiol Archived 2013-04-11 at the Wayback Machine
- ^ θεῖον Archived 2017-05-10 at the Wayback Motorcar, Henry George Liddell, Robert Scott, A Greek–English Lexicon
- ^ a b Patai, Saul, ed. (1974). The Chemical science of the Thiol Group. Part 1. London: Wiley. doi:x.1002/9780470771310. ISBN9780470771310.
- ^ Patai, Saul, ed. (1974). The Chemistry of the Thiol Group. Function 2. London: Wiley. doi:10.1002/9780470771327. ISBN9780470771327.
- ^ a b R. J. Cremlyn (1996). An Introduction to Organosulfur Chemistry. Chichester: John Wiley and Sons. ISBN978-0-471-95512-two.
- ^ Lexicon Reference: mercaptan Archived 2012-11-thirteen at the Wayback Car
- ^ a b Oxford American Dictionaries (Mac OS X Leopard).
- ^ Run across:
- Zeise, William Christopher (1834). "Mercaptanet, med bemaerkninger over nogle andre nye producter af svovelvinsyresaltene, som og af den tunge vinolie, ved sulfureter" [Mercaptan, with remarks on another new products of salts of ethyl hydrogen sulfate likewise equally of heavy oil of wine, past ways of hydrogen sulfide]. Kongelige Danske Videnskabers Selskabs Skrifter. fourth series (in Danish). 6: one–70. On p. 13 the give-and-take "mercaptan" is coined.
- German translation: Zeise, W. C. (1834). "Das Mercaptan, nebst Bermerkungen über einige neue Producte aus der Einwirkung der Sulfurete auf weinschwefelsaure Salze und auf das Weinöl" [Mercaptan together with comments on some new products from the effect of hydrogen sulfide on salts of ethyl sulfate ((C2Hfive)HSO4) and heavy oil of wine (a mixture of diethyl sulfate, diethyl sulfite, and polymerized ethylene)]. Annalen der Physik und Chemie. 2d series (in German). 31 (24): 369–431. From p. 378: " … nenne ich den vom Quecksilber aufgenommenen Stoff Mercaptum (von: Corpus mercurio captum) … " ( … I name the substance [that is] absorbed by mercury "mercaptum" (from: the trunk (substance) [that] has been absorbed by mercury) … )
- German translation is reprinted in:Zeise, Westward. C. (1834). "Das Mercaptan, nebst Bemerkungen über einige andere neue Erzeugnisse der Wirkung schwefelweinsaurer Salze, wie auch des schweren Weinöls auf Sulphurete". Journal für Praktische Chemie. 1 (1): 257–268, 345–356, 396–413, 457–475. doi:x.1002/prac.18340010154.
- Summarized in: Zeise, West. C. (1834). "Ueber das Mercaptan" [On mercaptan]. Annalen der Pharmacie. 11 (1): one–10. doi:x.1002/jlac.18340110102. Archived from the original on 2015-03-20.
- Zeise, William Christopher (1834). "Sur le mercaptan; avec des observations sur d'autres produits resultant de 50'action des sulfovinates ainsi que de 50'huile de vin, sur des sulfures metalliques" [On mercaptan; with observations on other products resulting from the action of sulfovinates [typically, ethyl hydrogen sulfate] likewise every bit oil of vino [a mixture of diethylsulfate and ethylene polymers] on metal sulfides]. Annales de Chimie et de Physique. 56: 87–97. Archived from the original on 2015-03-20. "Mercaptan" (ethyl thiol) was discovered in 1834 by the Danish professor of chemistry William Christopher Zeise (1789–1847). He called information technology "mercaptan", a wrinkle of "corpus mercurio captans" (mercury-capturing substance) [p. 88], because it reacted violently with mercury(Two) oxide ("deutoxide de mercure") [p. 92].
- The article in Annales de Chimie et de Physique (1834) was translated from the German language commodity: Zeise, W. C. (1834). "Das Mercaptan, nebst Bemerkungen über einige neue Producte aus der Einwirkung der Sulfurete auf weinschwefelsaure Salze und auf das Weinöl". Annalen der Physik und Chemie. 107 (27): 369–431. Bibcode:1834AnP...107..369Z. doi:x.1002/andp.18341072402. Archived from the original on 2015-03-20.
- ^ "Alkanethiols". Royal Lodge of Chemistry. Retrieved iv September 2019.
- ^ Lide, David R., ed. (2006). CRC Handbook of Chemical science and Physics (87th ed.). Boca Raton, FL: CRC Press. ISBN0-8493-0487-iii.
- ^ Andersen K. M.; Bernstein D. T. (1978). "Some Chemical Constituents of the Scent of the Striped Skunk (Mephitis mephitis)". Journal of Chemic Environmental. 1 (4): 493–499. doi:10.1007/BF00988589. S2CID 9451251.
- ^ Andersen K. Yard., Bernstein D. T.; Bernstein (1978). "1-Butanethiol and the Striped Skunk". Journal of Chemical Education. 55 (3): 159–160. Bibcode:1978JChEd..55..159A. doi:10.1021/ed055p159.
- ^ Andersen G. Yard.; Bernstein D. T.; Caret R. L.; Romanczyk L. J., Jr. (1982). "Chemical Constituents of the Defensive Secretion of the Striped Skunk (Mephitis mephitis)". Tetrahedron. 38 (13): 1965–1970. doi:10.1016/0040-4020(82)80046-X.
- ^ Wood West. F.; Sollers B. G.; Dragoo Thou. A.; Dragoo J. West. (2002). "Volatile Components in Defensive Spray of the Hooded Skunk, Mephitis macroura". Periodical of Chemical Environmental. 28 (nine): 1865–70. doi:10.1023/A:1020573404341. PMID 12449512. S2CID 19217201.
- ^ William F. Wood. "Chemical science of Skunk Spray". Dept. of Chemistry, Humboldt Country Academy. Archived from the original on October viii, 2010. Retrieved January 2, 2008.
- ^ Aldrich, T.B. (1896). "A Chemic Written report of the Secretion of the Anal Glands of Mephitis mephitiga (Mutual Skunk), with Remarks on the Physiological Properties of This Secretion". J. Exp. Med. ane (2): 323–340. doi:10.1084/jem.1.2.323. PMC2117909. PMID 19866801.
- ^ Lin, Dayu; Zhang, Shaozhong; Cake, Eric; Katz, Lawrence C. (2005). "Encoding social signals in the mouse principal olfactory seedling". Nature. 434 (7032): 470–477. Bibcode:2005Natur.434..470L. doi:10.1038/nature03414. PMID 15724148. S2CID 162036.
- ^ Duan, Xufang; Block, Eric; Li, Zhen; Connelly, Timothy; Zhang, Jian; Huang, Zhimin; Su, Xubo; Pan, Yi; et al. (2012). "Crucial role of copper in detection of metal-coordinating odorants". Proc. Natl. Acad. Sci. UsA. 109 (9): 3492–3497. Bibcode:2012PNAS..109.3492D. doi:ten.1073/pnas.1111297109. PMC3295281. PMID 22328155.
- ^ "Copper cardinal to our sensitivity to rotten eggs' foul olfactory property". chemistryworld.com. Archived from the original on 10 May 2017. Retrieved 3 May 2018.
- ^ Roberts, J. S., ed. (1997). Kirk-Othmer Encyclopedia of Chemical Engineering science. Weinheim: Wiley-VCH. [ folio needed ]
- ^ Luo, Y.-R.; Cheng, J.-P. (2017). "Bond Dissociation Energies". In J. R. Rumble (ed.). Handbook of Chemistry and Physics. CRC Press.
{{cite book}}: CS1 maint: uses authors parameter (link) - ^ Human being, Pascal P. "Sulfur-33 NMR references". www.pascal-homo.com. Archived from the original on 23 August 2017. Retrieved 3 May 2018.
- ^ John S Roberts, "Thiols", in Kirk-Othmer Encyclopedia of Chemical Technology, 1997, Wiley-VCH, Weinheim. doi:10.1002/0471238961.2008091518150205.a01
- ^ Speziale, A. J. (1963). "Ethanedithiol". Organic Syntheses. ; Collective Volume, vol. 4, p. 401 .
- ^ Urquhart, G. G.; Gates, J. West. , Jr.; Connor, Ralph (1941). "due north-Dodecyl Mercaptan". Org. Synth. 21: 36. doi:10.15227/orgsyn.021.0036.
- ^ South. R. Wilson, G. Chiliad. Georgiadis (1990). "Mecaptans from Thioketals: Cyclododecyl Mercaptan". Organic Syntheses. ; Collective Book, vol. 7, p. 124 .
- ^ E. Jones and I. Grand. Moodie (1990). "ii-Thiophenethiol". Organic Syntheses. ; Collective Volume, vol. 6, p. 979 .
- ^ Melvin S. Newman and Frederick Westward. Hetzel (1990). "Thiophenols from Phenols: ii-Naphthalenethiol". Organic Syntheses. ; Commonage Volume, vol. 6, p. 824 .
- ^ Ernest 50. Eliel, Joseph E. Lynch, Fumitaka Kume, and Stephen V. Frye (1993). "Chiral 1,three-oxathiane from (+)-Pulegone: Hexahydro-4,four,7-trimethyl-4H-1,iii-benzoxathiin". Organic Syntheses.
{{cite periodical}}: CS1 maint: multiple names: authors list (link); Collective Volume, vol. 8, p. 302 - ^ Kazem-Rostami, Masoud; Khazaei, Ardeshir; Moosavi-Zare, Ahmad; Bayat, Mohammad; Saednia, Shahnaz (2012). "Novel One-Pot Synthesis of Thiophenols from Related Triazenes under Mild Conditions". Synlett. 23 (13): 1893–1896. doi:x.1055/s-0032-1316557.
- ^ Leuckart, Rudolf (1890). "Eine neue Methode zur Darstellung aromatischer Mercaptane" [A new method for the preparation of aromatic mercaptans]. Journal für Praktische Chemie. 2nd serial (in German). 41: 179–224. doi:10.1002/prac.18900410114.
- ^ M. E. Alonso and H. Aragona (1978). "Sulfide Synthesis in Preparation of Unsymmetrical Dialkyl Disulfides: Sec-butyl Isopropyl Disulfide". Org. Synth. 58: 147. doi:10.15227/orgsyn.058.0147.
{{cite journal}}: CS1 maint: uses authors parameter (link) - ^ Akhmadullina, A. One thousand.; Kizhaev, B. Five.; Nurgalieva, G. M.; Khrushcheva, I. Grand.; Shabaeva, A. South.; et al. (1993). "Heterogeneous catalytic demercaptization of light hydrocarbon feedstock". Chemistry and Engineering science of Fuels and Oils. 29 (3): 108–109. doi:10.1007/bf00728009. S2CID 97292021. Archived from the original on 2011-08-15.
- ^ Roy, Kathrin-Maria (2005). "Thiols and Organic Sulphides". Ullmann'due south Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:x.1002/14356007.a26_767.
- ^ Stubbe, JoAnne; Nocera, Daniel Thou.; Yee, Cyril S.; Chang, Michelle C. Y. (2003). "Radical Initiation in the Class I Ribonucleotide Reductase: Long-Range Proton-Coupled Electron Transfer?". Chem. Rev. 103 (half dozen): 2167–2202. doi:10.1021/cr020421u. PMID 12797828.
- ^ Hofstetter, Dustin; Nauser, Thomas; Koppenol, Willem H. (2010). "Hydrogen Exchange Equilibria in Glutathione Radicals: Charge per unit Constants". Chem. Res. Toxicol. 23 (10): 1596–1600. doi:10.1021/tx100185k. PMC2956374. PMID 20882988.
- ^ Koch, Cameron J.; Parliament, Matthew B.; Brownish, J. Martin; Urtasun, Raul C. (2010). "Chemical Modifiers of Radiation Response". Leibel and Phillips Textbook of Radiation Oncology. Elsevier. pp. 55–68. doi:10.1016/b978-i-4160-5897-7.00004-four. ISBN978-1-4160-5897-7.
Sulfhydryls are scavengers of gratuitous radicals, protecting chemical impairment induced by either ionizing radiations or alkylating agents.
- ^ Reece, Urry; et al. (2011). Campbell Biology (Ninth ed.). New York: Pearson Benjamin Cummings. pp. 65, 83.
- ^ Malle, Eastward (2007). "Myeloperoxidase: a target for new drug development?". British Journal of Pharmacology. 152 (vi): 838–854. doi:x.1038/sj.bjp.0707358. PMC2078229. PMID 17592500.
- ^ "Understanding Owls – The Owls Trust". theowlstrust.org. Archived from the original on 5 February 2018. Retrieved iii May 2018.
External links [edit]
- Mercaptans (or Thiols) at The Periodic Tabular array of Videos (University of Nottingham)
- Applications, Properties, and Synthesis of ω-Functionalized n-Alkanethiols and Disulfides – the Building Blocks of Self-Assembled Monolayers by D. Witt, R. Klajn, P. Barski, B.A. Grzybowski at Northwestern Academy.
- Mercaptan, by The Columbia Electronic Encyclopedia.
- What is Mercaptan?, by Columbia Gas of Pennsylvania and Maryland.
- What Is the Worst Smelling Chemical?, by Well-nigh Chemistry.
Source: https://en.wikipedia.org/wiki/Thiol
0 Response to "The Compound Ch3 - Ch2 - Sh Is in the Organic Family Known as"
Post a Comment